Abstract
Introduction: WM, an indolent but incurable B-cell neoplasm, has two critical survival pathways; the ubiquitin proteasome degradation system (UPS) and B-cell receptor signalling via hyperactivation of Bruton's tyrosine kinase (BTK). Ixazomib, an orally bioavailable proteasome inhibitor, targets the UPS and ibrutinib inhibits BTK signaling. Both ixazomib and ibrutinib have demonstrated anti-WM activity however, most of the responses are limited to partial responses (PR) with very few patients (pts) achieving complete response (CR). Dual targeting of the major survival pathways in WM may augment the depth of response and change the natural history of WM. We report preliminary data from a phase 2 trial evaluating the efficacy of ixazomib and ibrutinib in pts with newly diagnosed (NDWM) as well as relapsed/refractory WM (RRWM).
Methods: This single arm, phase 2 trial (NCT03506373) enrolled 24 pts with WM. Key eligibility criteria included: NDWM or RRWM; pts previously treated with ibrutinib were allowed so long as they did not develop disease progression (PD) while on ibrutinib or within 6 months of stopping ibrutinib. Pts needed to have indications to initiate treatment for WM as per the IWWM-7 consensus and measurable disease defined as IgM paraprotein, measurable lymphadenopathy, and bone marrow infiltration >10%. Pts needed to have an absolute neutrophil count ≥1.0x109, platelets ≥75x109, Hemoglobin >9.0 g/dL, creatinine clearance ≥30 mL/min, and ECOG performance status ≤2. Pts received ixazomib 4 mg PO on days 1, 8 and 15 and ibrutinib 420 mg PO daily in 4-weekly cycles for a maximum of 24 cycles or PD (if sooner). An interim analysis based on a two-stage Simon optimum design was planned to evaluate primary endpoint of CR rate with a null hypothesis in this patient population being at most 5%.
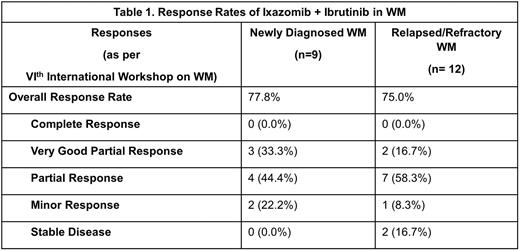
Results: Of 24 pts enrolled, 21 pts were analyzed (ineligible: 1, screen failures: 2). 9 (42.9%) had NDWM and 12 (57.1%) had RRWM. Median follow-up time was 18.2 months (range: 9.5-45.1). The median age was 72 years (range: 54-79). The median ISSWM score was 1.0 (range: 0.0-3.0). 15 pts (71.4%) had mutated (mut) MYD88L265P, 6 pts (28.6%) had CXCR4mut, with 1 pt (4.8%) having MYD88L265P wild type (wt) and CXCR4mut. Pts with RRWM had received a median of 2 (range: 1-4) prior lines of therapy; 10 (83.3%) received rituximab, 4 (33.3%) received bortezomib, 6 (50.0%) received alkylator-based therapy. At the time of this analysis, 3 pts had completed the planned two years of therapy. 18 pts did not complete two years of therapy: 6 due to adverse event (AE), 4 due to PD, 2 due to pt withdrawal, 3 due to other reasons, and 3 are still on treatment. The overall response rate (ORR) was 76.2% (n=16) with 0 CRs, 5 (23.8%) VGPRs, 11 (52.4%) PRs, 3 (14.3%) MRs, and 2 (9.5%) SD. This led to a clinical benefit rate (CBR) of 100.0% (n=21). Differences in ORR between NDWM and RRWM are shown in Table 1. In 8 pts that were MYD88mut/CXCRwt the ORR was 75.0%, 1 pt that was MYD88wt/CXCR4wt and 1 pt that was MYD88wt/CXCR4mut both had an objective response. The ORR was 83.3% (5/6 with ≥PR) in CXCR4mut pts. The median time to progression (TTP) was 25.7 months (95% CI: 15.9, NE). The most common AEs were anemia (81%), fatigue (76%), nausea (67%), thrombocytopenia (52%), and vomiting (48%). Grade 3/4 AEs included neutropenia (3 pts) and, anemia, hypertension, hypoxia, peripheral sensory neuropathy, and lung infection in 2 pts each.
Conclusion: The study failed to meet the primary endpoint as no patients achieved a CR. However, >50% of the pt population had RRWM and had received a median of two prior lines of therapy. Nonetheless, the combination of time-limited ibrutinib and ixazomib led to a clinically meaningful high ORR of 76.2%, a CBR of 100% and a median TTP of 25.7 months across biologic subtypes. Toxicities were manageable although 6 patients discontinued due to low-grade ongoing AEs. Continued evaluation of combined proteasome and BTK inhibition is warranted for the treatment of WM.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal